Related Products
ACTIVE INGREDIENT: LETROZOLE
TABLET COUNT: 50 TABLETS
HALF-LIFE: 2 DAYS
CLASSIFICATION: AROMATASE INHIBITOR
DOSAGE: 0.25-2.5 MG PER DAY
ACNE: LOW
WATER RETENTION: LOW
HEPATOTOXICITY: LOW
AROMATIZATION: NONE
ACTIVE INGREDIENT: CHLORODEHYDROMETHYLTESTOSTERONE (TURINABOL)
TABLET COUNT: 50 TABLETS
HALF-LIFE: 16 HOURS
CLASSIFICATION: ANABOLIC STEROID
DOSAGE: 20-50 MG/DAY
ACNE: POSSIBLE
WATER RETENTION: LOW
HEPATOTOXICITY: MODERATE
AROMATIZATION: NONE
ACTIVE INGREDIENT: FLUOXYMESTERONE (HALOTESTIN)
HALF-LIFE: 9 HOURS
CLASSIFICATION: ANABOLIC ANDROGENIC STEROID
DOSAGE: 10-20MG/DAY
ACNE: HIGH
WATER RETENTION: LOW
HEPATOTOXICITY: HIGH
AROMATIZATION: NONE
ACTIVE HALF-LIFE: 2-3 days
CLASSIFICATION: Anabolic Steroid
DOSAGE: Men – 50mg-100mg/day
ACNE: Low
WATER RETENTION: No
HBR: No
HEPATOTOXICITY: No
AROMATIZATION: No
MANUFACTURER: Beligas Pharmaceutical – Int’l
WAREHOUSE: International Warehouse 5
SUBSTANCE: Methenolone Acetate
ACTIVE INGREDIENT: SILDENAFIL CITRATE
TABLET COUNT: 50 TABLETS
HALF-LIFE: 4 HOURS
CLASSIFICATION: PDE5 INHIBITOR
DOSAGE: 25-100 MG
ACNE: NONE
WATER RETENTION: LOW
HEPATOTOXICITY: LOW
AROMATIZATION: NONE
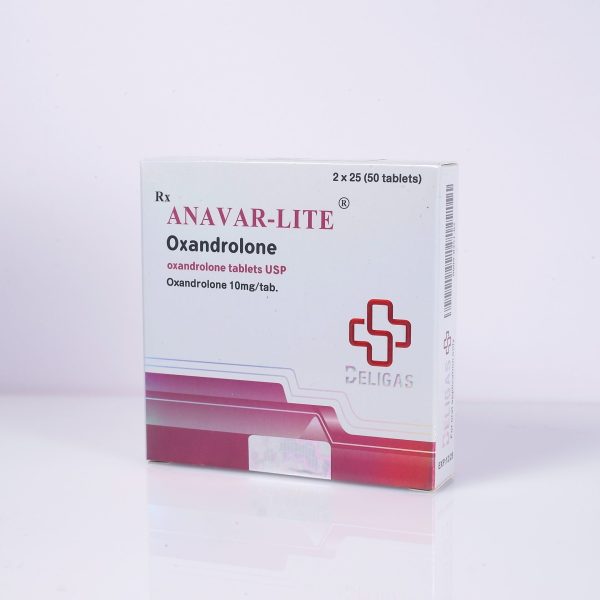
ACTIVE INGREDIENT: OXANDROLONE (ANAVAR)
TABLET COUNT: 50 TABLETS
HALF-LIFE: 9-12 HOURS
CLASSIFICATION: ANABOLIC STEROID
DOSAGE: 20-80 MG/DAY
ACNE: POSSIBLE
WATER RETENTION: LOW
HEPATOTOXICITY: MODERATE
AROMATIZATION: NONE
ACTIVE INGREDIENT: METHYLDROSTANOLONE
TABLET COUNT: 100 TABLETS
HALF-LIFE: 8-12 HOURS
CLASSIFICATION: ANABOLIC STEROID
DOSAGE: 10-20 MG PER DAY
ACNE: MODERATE TO HIGH
WATER RETENTION: LOW
HEPATOTOXICITY: HIGH
AROMATIZATION: NONE
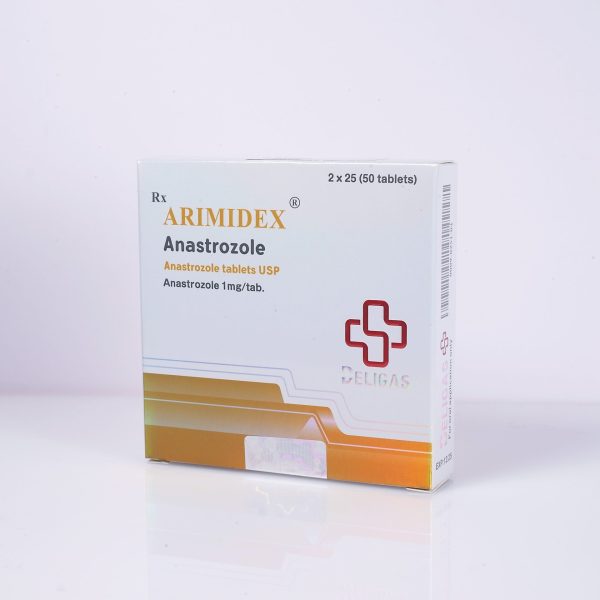
ACTIVE INGREDIENT: ANASTROZOLE (ARIMIDEX)
TABLET COUNT: 50 TABLETS
HALF-LIFE: 46.8 HOURS
CLASSIFICATION: AROMATASE INHIBITOR
DOSAGE: 0.5-1 MG/DAY
ACNE: LOW
WATER RETENTION: LOW
HEPATOTOXICITY: LOW
AROMATIZATION: PREVENTS
ACTIVE INGREDIENT: MESTEROLONE (PROVIRON)
TABLET COUNT: 50 TABLETS
HALF-LIFE: 12-13 HOURS
CLASSIFICATION: ANDROGEN; ANABOLIC STEROID
DOSAGE: 50-150 MG/DAY
ACNE: POSSIBLE
WATER RETENTION: LOW
HEPATOTOXICITY: LOW
AROMATIZATION: NONE